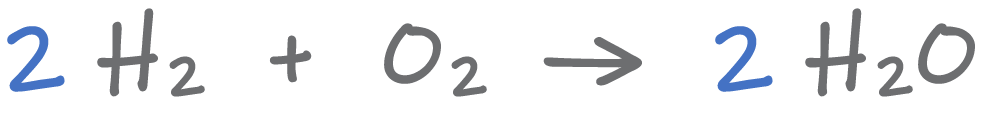This is a lesson summary. The full lesson can be viewed by purchasing an online course subscription.
Learning Objective
In this lesson we will learn how to write chemical equations in a way that reflects the law of conservation of mass.
Learning Outcomes
By the end of this lesson you will be able to:
- Explain how the law of conservation of mass applies to chemical reactions.
- Write balanced formula equations to represent chemical reactions.
- Include states of matter in formula equations.
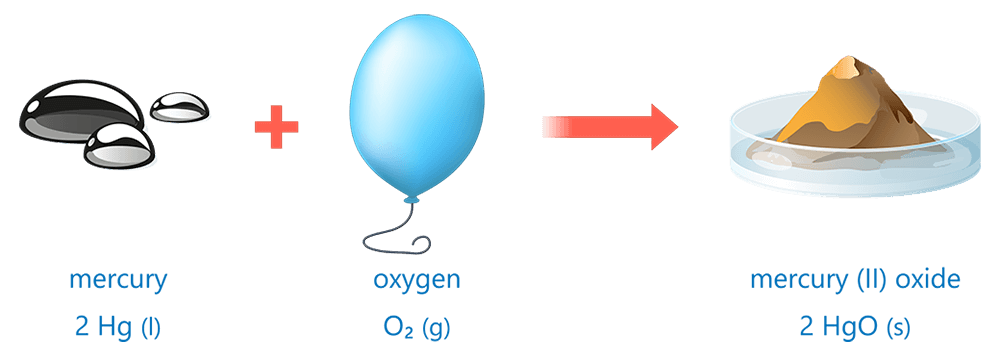
(Image: designua, Adobe Stock)
Lesson Summary
- A word equation shows the chemical names of all reactants and products involved in a chemical reaction.
- A formula equation shows the chemical formulas of all reactants and products involved in a chemical reaction.
- Formula equations may also include the states of matter of reactants and products in brackets after each chemical formula.
- (s) represents solids.
- (l) represents liquids.
- (g) represents gases.
- (aq) represents aqueous solutions.
- The law of conservation of mass states that matter is neither created nor destroyed.
- Therefore, for chemical reactions, the total mass of the products equals the total mass of the reactants and the total numbers of each type of atom are the same before and after the reaction has occurred.
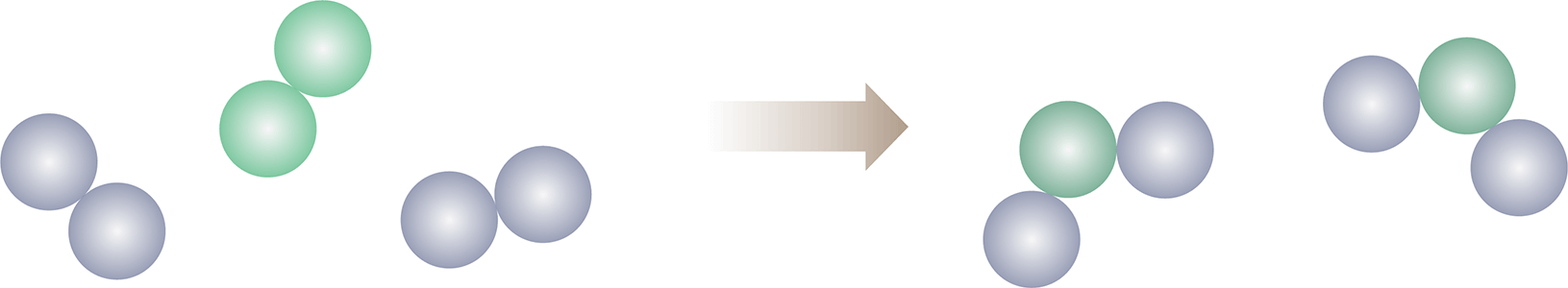
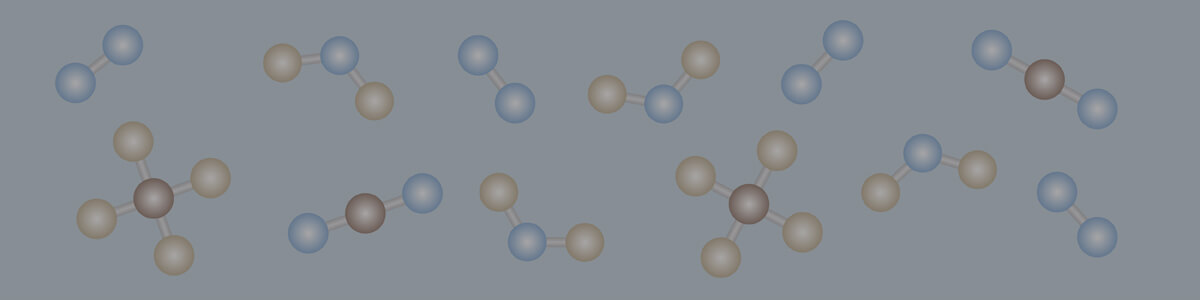
- A balanced chemical equation contains equal numbers of each type of atom on both sides of the equation and therefore demonstrates conservation of mass.
- An unbalanced chemical equation does not contain equal numbers of each type of atom on both sides of the equation and therefore does not demonstrate conservation of mass.
- To reflect the conservation of mass in a chemical reaction, an unbalanced chemical equation can be balanced by adding coefficients in front of chemical formulas of reactants and products.
- These coefficients reflect the ratios of each reactant and product involved in the reaction.
- Steps for writing a balanced chemical equation:
- Write the formula equation showing all reactants and products.
- Tally up the total numbers of each type of atom for both sides of the equation.
- Place coefficients in front of reactants and products until there are equal numbers of each type of atom on both sides of the equation.