This is a lesson summary. The full lesson can be viewed by purchasing an online course subscription.
Learning Objective
In this lesson we will learn how the products of chemical reactions can often be predicted, by classifying reactions into different types, based on the way atoms are rearranged.
Learning Outcomes
By the end of this lesson you will be able to:
- Identify the following types of chemical reactions:
- Combination
- Decomposition
- Single displacement
- Double displacement (precipitation and neutralisation)
- Predict the products of the following types of chemical reactions:
- Single displacement
- Precipitation
- Neutralisation
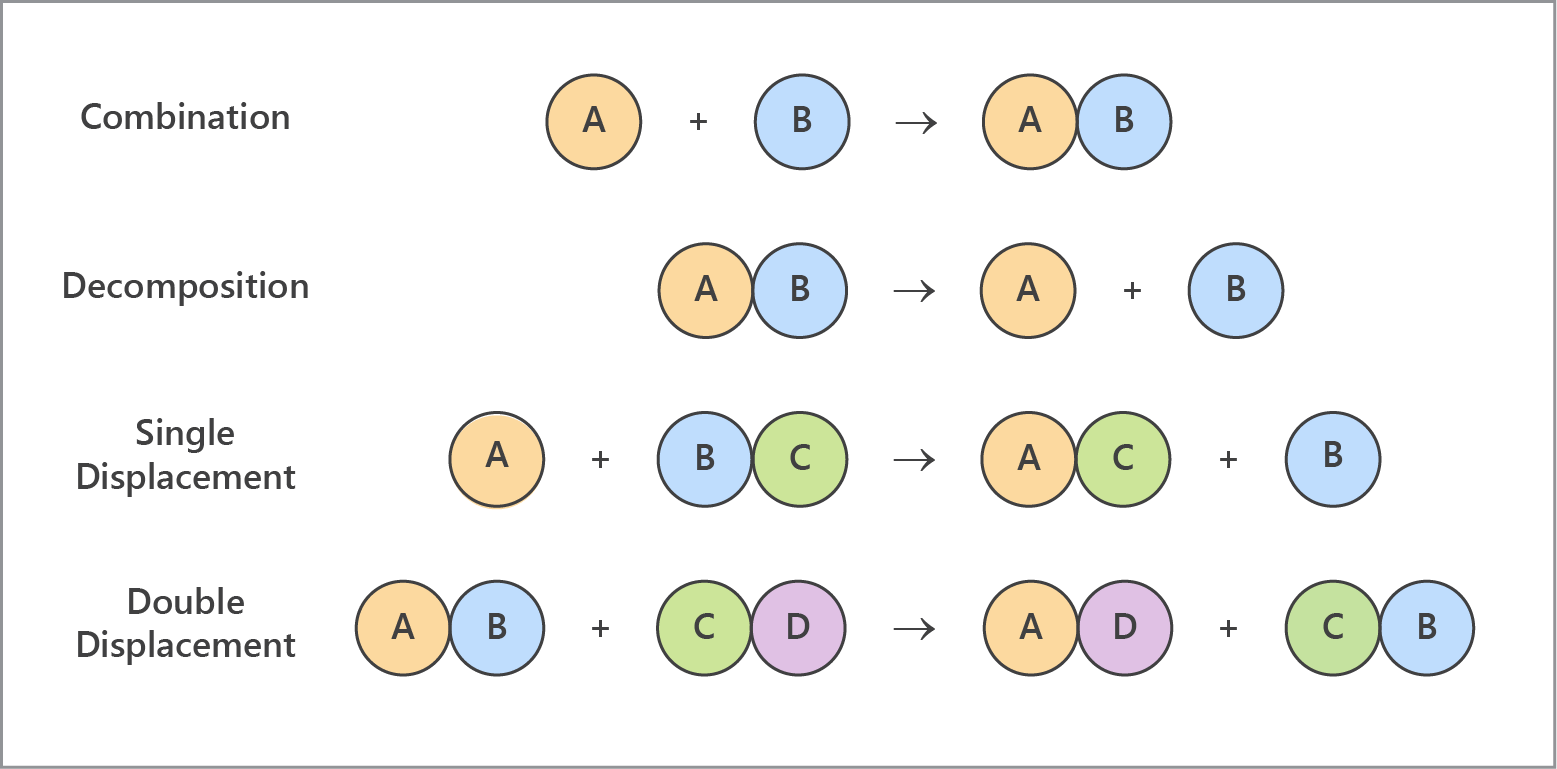
Lesson Summary
- Chemical reactions can be classified based on the way atoms and molecules are rearranged during the reaction.
- Classifying chemical reactions can make it possible to predict their products.
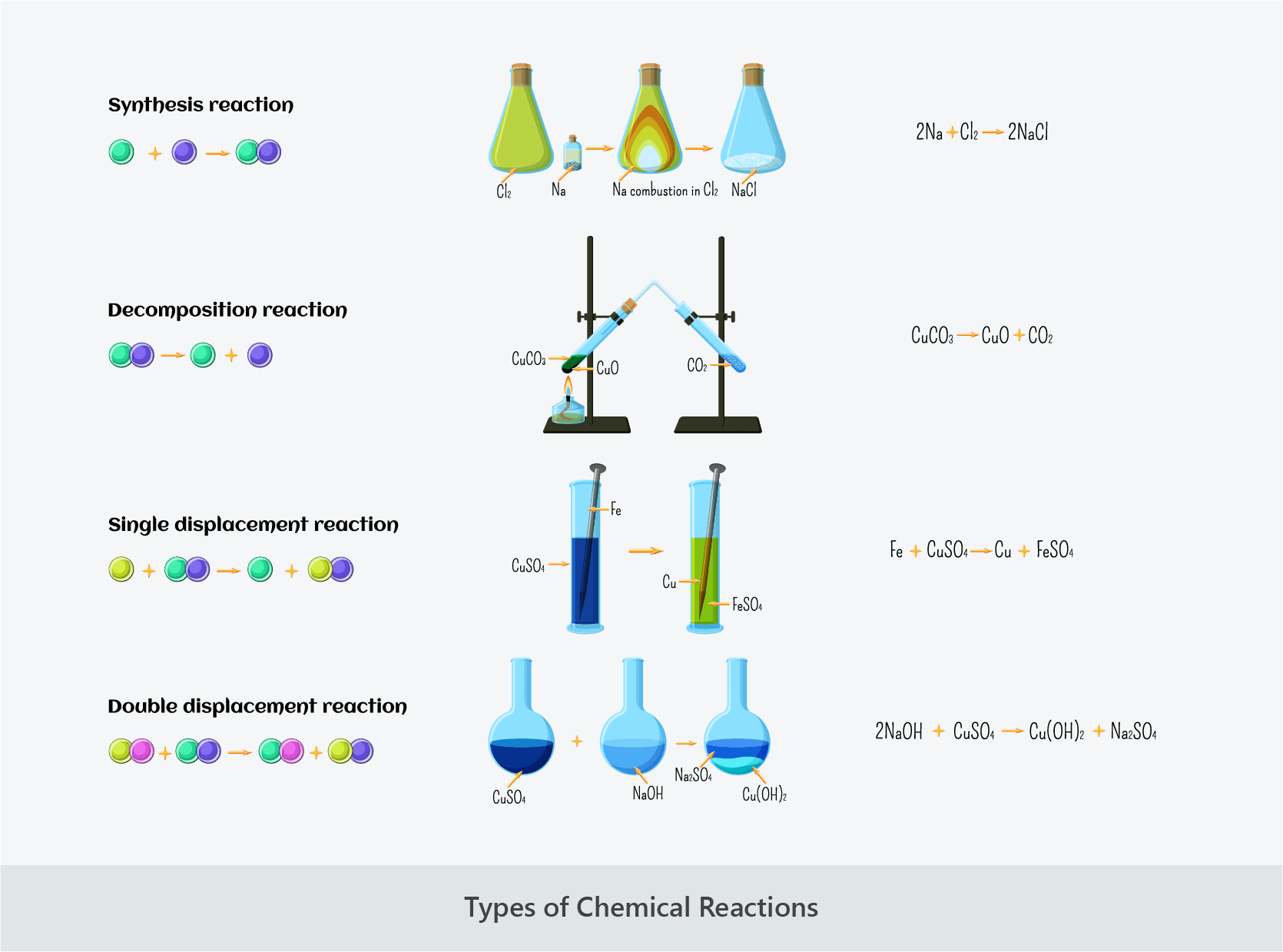
- Combination reactions involve two or more reactants combining to form a single product:
- A + B → AB
- Decomposition reactions involve a single reactant breaking down to form two or more products:
- AB → A + B
- Single displacement reactions involve an element and a compound reacting to form a different element and compound:
- A + BC → AC + B
- Double displacement reactions involve two compounds reacting to form two different compounds:
- AB + CD → AD + CB
(Image: Inna, Adobe Stock)
(Header image: vadimborkin, Adobe Stock)
