This is a lesson summary. The full lesson can be viewed by purchasing an online course subscription.
Learning Objective
In this lesson we will learn about the main subatomic particles which make up atoms.
Learning Outcomes
By the end of this lesson you will be able to:
- Describe the properties of protons, neutrons and electrons.
- Describe the arrangement of protons, neutrons and electrons in an atom.
- Describe the distribution of mass and charge within an atom.
(Image: nobeastsofierce, Adobe Stock)
Lesson Summary
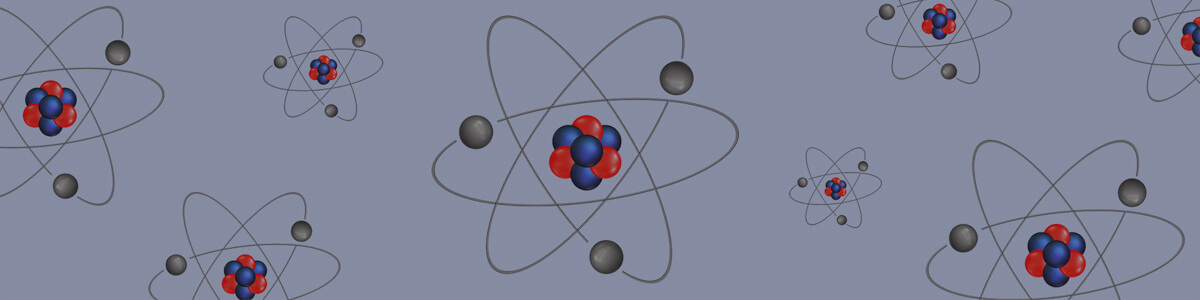
- Atoms are composed of subatomic particles called protons, neutrons and electrons.
- Protons have a mass of 1 atomic mass unit and a positive charge (+1).
- The number of protons determines the type of atom.
- Neutrons have a mass of 1 atomic mass unit and no charge.
- Electrons have a mass of 1/1800 of an atomic mass unit and a negative charge (–1).
- Protons and neutrons form a dense cluster at the centre of an atom, called a nucleus.
- Electrons move around the nucleus, forming an electron cloud.
| Particle / Structure | Location | Charge | Mass (atomic mass units) |
| Proton | Nucleus | +1 | 1 |
| Neutron | Nucleus | 0 | 1 |
| Electron | Electron Cloud | –1 | 1/1800 |
| Nucleus | Centre of atom | positive (amount depends on atom) |
(depends on atom) |
| Electron Cloud | Around the nucleus | negative (amount depends on atom) |
(depends on atom, but negligible) |
| Atom | – | 0 | (depends on atom, approximately same as nucleus) |
Summary of atomic structure
(Header image: geralt, Pixabay)