This is a lesson summary. The full lesson can be viewed by purchasing an online course subscription.
Learning Objective
In this lesson we will learn how unstable isotopes can undergo different types of nuclear decay.
Learning Outcomes
By the end of this lesson you will be able to:
- Describe alpha, beta and gamma decay.
- Predict the products of different types of nuclear decay.
- Define half life and calculate how much of a radioactive substance will remain after a given amount of time.
- Compare the penetrating power of alpha, beta and gamma radiation.
- Define radiation dose and give examples of natural and artificial sources of radiation.
(Image: Aliaksandr Marko, Adobe Stock)
Lesson Summary
- Some atoms are unstable due to unbalanced forces between nuclear particles – protons and neutrons.
- These isotopes, known as radioisotopes, undergo spontaneous nuclear decay.
- Nuclear decay involves the emission of nuclear radiation from the nuclei of radioisotopes.
- There are three main types of nuclear decay – alpha, beta and gamma.
- Alpha decay is the ejection of alpha particles (helium nuclei) from atoms.
- It decreases the atomic number and therefore results in the formation of a new element (transmutation). The mass number also decreases.
- Beta decay is the ejection of beta particles (electrons) from atoms.
- These electrons form when a neutron is converted into a proton and an electron.
- Beta decay increases the atomic number and therefore results in the formation of a new element. The mass number remains the same.
- Gamma decay is the emission of gamma rays (electromagnetic waves) from atoms.
- It does not change the atomic number (or mass number), therefore the type of element stays the same.
- The half life of a radioisotope is the time it takes for half of its nuclei to undergo nuclear decay.
- All types of radiation are damaging to living things, but each penetrates materials to different extents.
- Gamma radiation is the most penetrating and alpha radiation is the least penetrating.
- Radiation dose is a measure of how much radiation is absorbed by a substance or individual.
- Sources of radiation can be natural or artificial, with natural sources accounting for the majority of radiation absorbed by humans.
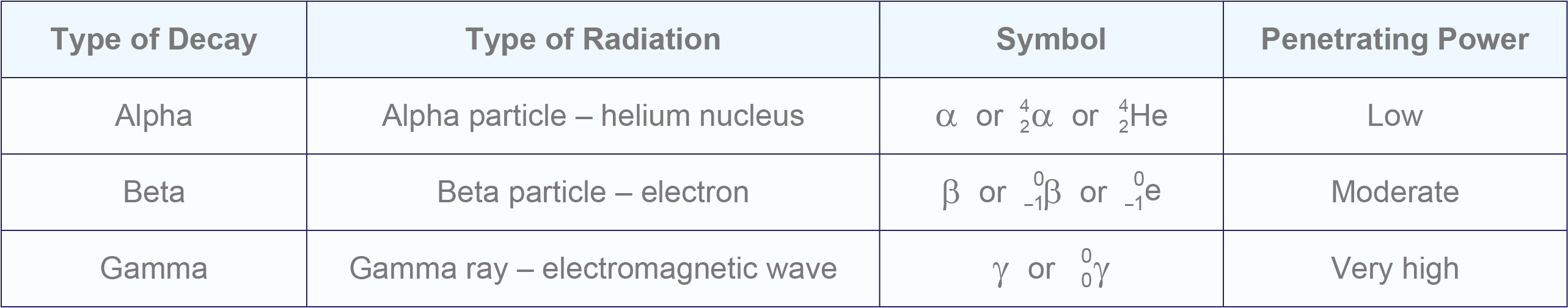
Summary of the types of nuclear decay.
(Header image: bluedesign, Adobe Stock)
