This is a lesson summary. The full lesson can be viewed by purchasing an online course subscription.
Learning Objective
In this lesson we will learn how matter is classified based on its macroscopic and microscopic properties.
Learning Outcomes
By the end of this lesson you will be able to:
- Define matter and explain how it is composed of particles.
- Distinguish between a pure substance and a mixture.
- Distinguish between a homogeneous mixture and heterogeneous mixture.
- Explain how matter can be observed at the macroscopic and microscopic level.
- Distinguish between solutions, colloids and coarse mixtures.

(Image: Devanath, Pixabay)
Lesson Summary
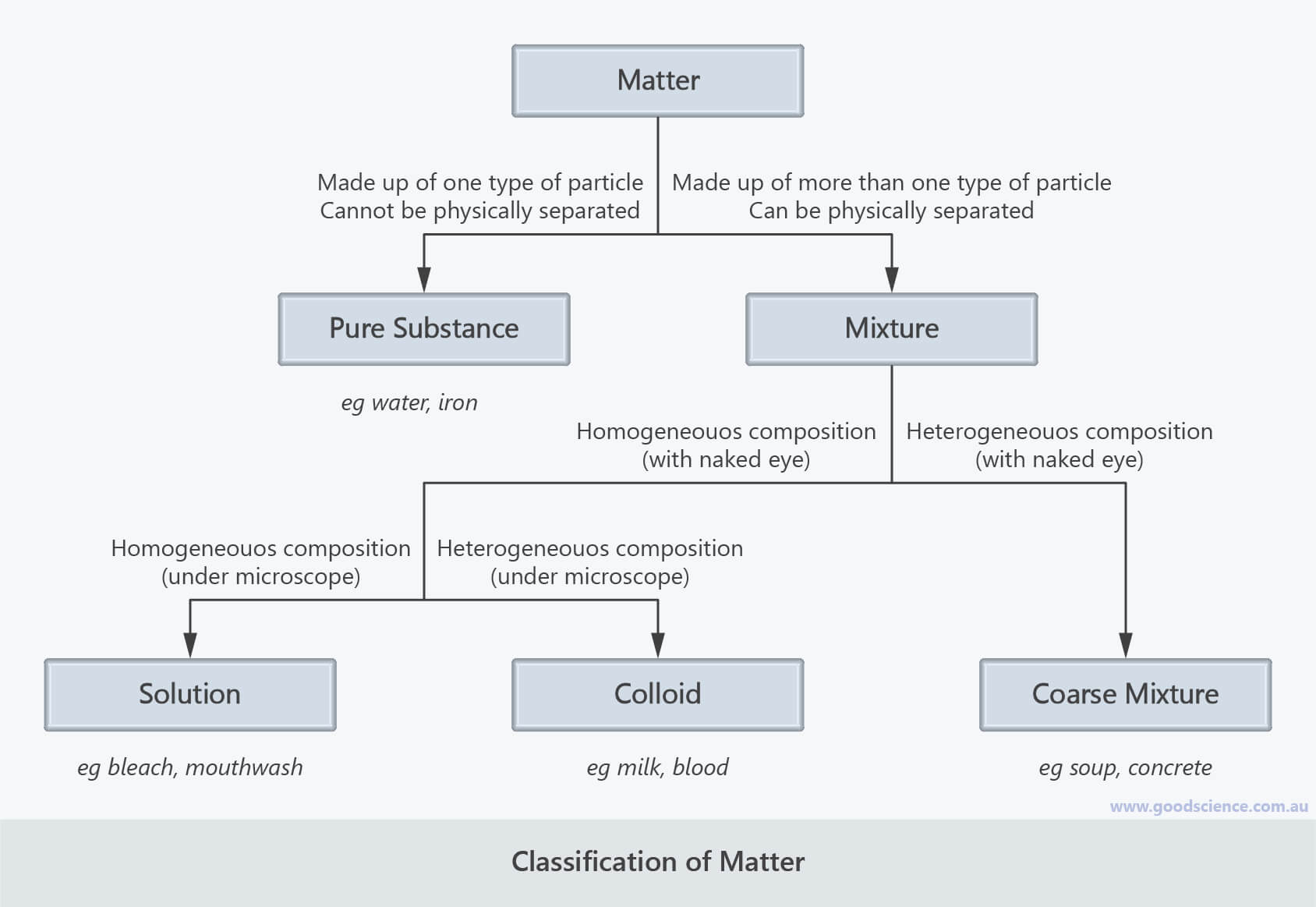
- Matter is anything that takes up space and has mass.
- Matter can be divided into pure substances and mixtures.
- Pure substances are made up of one type of particle and cannot be physically separated into other substances.
- Mixtures are made up of more than one type of particle and can be physically separated into other substances.
- Mixtures can be classified as homogeneous or heterogeneous.
- Homogeneous mixtures consist of a single phase and have a uniform composition.
- Heterogeneous mixtures consist of two or more phases and do not have a uniform composition.
- Solutions are mixtures that are homogeneous at the macroscopic and microscopic level.
- Coarse mixtures are mixtures that are heterogeneous at the macroscopic and microscopic level.
- Colloids are mixtures that are homogeneous at the macroscopic level but heterogeneous at the microscopic level.
- Colloids can be distinguished from solutions as they are opaque whereas solutions are transparent; colloids can also be spun into different phases whereas solutions cannot.
(Header image: PublicDomainPictures, Pixabay)
