This is a lesson summary. The full lesson can be viewed by purchasing an online course subscription.
Learning Objective
In this lesson we will learn how the energy, arrangement and movement of particles in solids, liquids and gases determines their different properties.
Learning Outcomes
By the end of this lesson you will be able to:
- Describe the particle model of matter.
- Describe the energy, arrangement and movement of particles in solids, liquids and gases.
- Use the particle model to explain the properties of solids, liquids and gases.
(Image: designua, Adobe Stock)
Lesson Summary
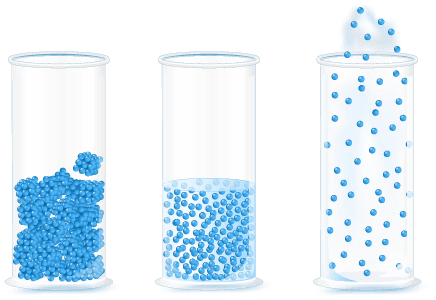
- The particle model describes the energy, arrangement and movement of particles in solids, liquids and gases.
- Particles in solids:
- ▸ Have low kinetic energy.
- ▸ Are very strongly attracted to each other.
- ▸ Are closely packed, in a fixed arrangement.
- ▸ Move by vibrating.
- Particles in liquids:
- ▸ Have medium kinetic energy.
- ▸ Are strongly attracted to each other.
- ▸ Are closely packed, in a free arrangement.
- ▸ Move by sliding past one another.
- Particles in gases:
- ▸ Have high kinetic energy.
- ▸ Are not attracted to each other.
- ▸ Are spaced far apart, in a free arrangement.
- ▸ Move in all directions.
- The particle model accounts for the different properties of solids, liquids and gases, such as shape, compressibility and density.

(Image: PiccoloNamek, Wikimedia Commons)
(Header image: geralt, Pixabay)
