This is a lesson summary. The full lesson can be viewed by purchasing an online course subscription.
Learning Objective
In this lesson we will learn how ions and ionic compounds are named.
Learning Outcomes
By the end of this lesson you will be able to:
- Name positive and negative monatomic ions.
- Give examples of metals that form more than one type of ion, and show how these ions are differentiated.
- Look up the names and formulas of polyatomic ions on a valency table.
- Determine the names of ionic compounds from their formulas.

(Image: antoine2k, Adobe Stock)
Lesson Summary
- Monatomic ions are ions that contain one type of atom.
- Polyatomic ions are ions that contain more than one type of atom.
- Monatomic positive ions have the same name as the metal atoms they are formed from.
- For metals that form more than one type of ion, Roman numerals corresponding to the size of the positive charge are used to distinguish between the different ions.
- Monatomic negative ions have the same name as the non-metal atoms they are formed from, except the last part of their name is changed to “-ide”.
- The names of polyatomic ions cannot be predicted from their formulas, but can be looked up in a valency table.
- The names of ionic compounds are derived by joining the names of the positive and negative ions they are composed of.
- The positive ion forms the first part of the compound name.
- The negative ion forms the second part of the compound name.
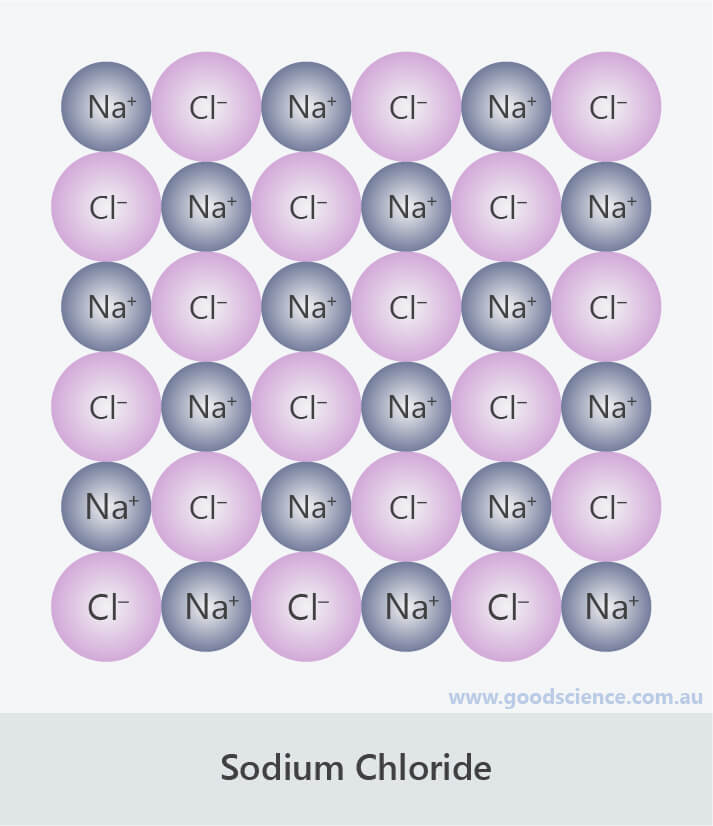
Sodium chloride lattice, showing sodium and chloride ions.