This is a lesson summary. The full lesson can be viewed by purchasing an online course subscription.
Learning Objective
In this lesson we will learn how chemical reactions can be classified, based on the way atoms are rearranged, or according to the energy changes that take place. We will also learn how to test for oxygen, hydrogen and carbon dioxide gas produced during chemical reactions.
Learning Outcomes
By the end of this lesson you will be able to:
- Describe the general rearrangement of atoms during combination, decomposition, displacement and combustion reactions.
- Distinguish between exothermic and endothermic chemical reactions.
- Distinguish between spontaneous and non-spontaneous chemical reactions.
- Describe how to test for the presence of oxygen, hydrogen and carbon dioxide gas.

(Image: Life & chemicals, Adobe Stock)
Lesson Summary
- There are many different chemical reactions, which can be categorised into general types, based on the way atoms and molecules are rearranged.
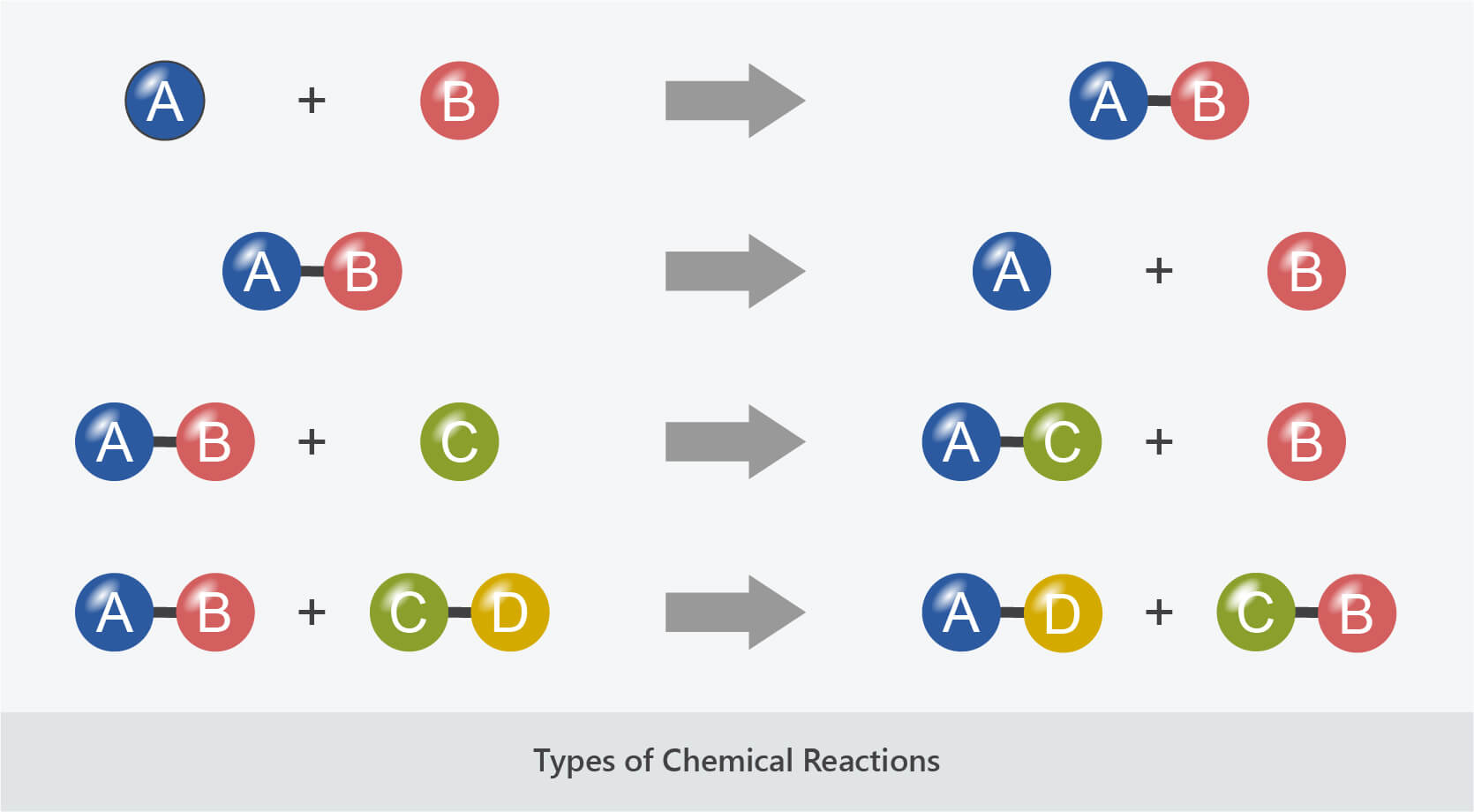
- Combination reactions involve two or more reactants combining to form a single product.
- They can be represented as: A + B → AB
- Decomposition reactions involve a single reactant breaking down to form two or more products.
- They can be represented as: AB → A + B
- Displacement reactions involve the displacing or swapping of atoms.
- Single displacement reactions can be represented as: A + BC → AC + B
- Double displacement reactions can be represented as: AB + CD → AD + CB
- Combustion reactions involve the reaction of hydrocarbons with oxygen.
- They can be represented as: CxHy + O2 → CO2 + H2O
- Chemical reactions can also be classified based on energy changes that take place.
- An exothermic reaction releases heat energy into the surrounding environment.
- An endothermic reaction absorbs heat energy from the surrounding environment.
- Chemical reactions can also be classified based on whether they require a constant input of energy or not.
- A spontaneous reaction does not require a constant supply of energy to continue.
- A non-spontaneous reaction does require a constant supply of energy to continue.
- A lit splint can be used to test for and distinguish between oxygen, hydrogen and carbon dioxide gas.
- Oxygen gas will cause a flame to burn brighter.
- Hydrogen gas will cause a “pop” sound.
- Carbon dioxide gas will extinguish a flame.
- The presence of carbon dioxide can also be confirmed if the gas turns limewater cloudy, when bubbled through it.
(Image: Daniele Pugliesi, Wikimedia Commons)
(Header image: Spiff, Wikimedia Commons)
