This is a lesson summary. The full lesson can be viewed by purchasing an online course subscription.
Learning Objective
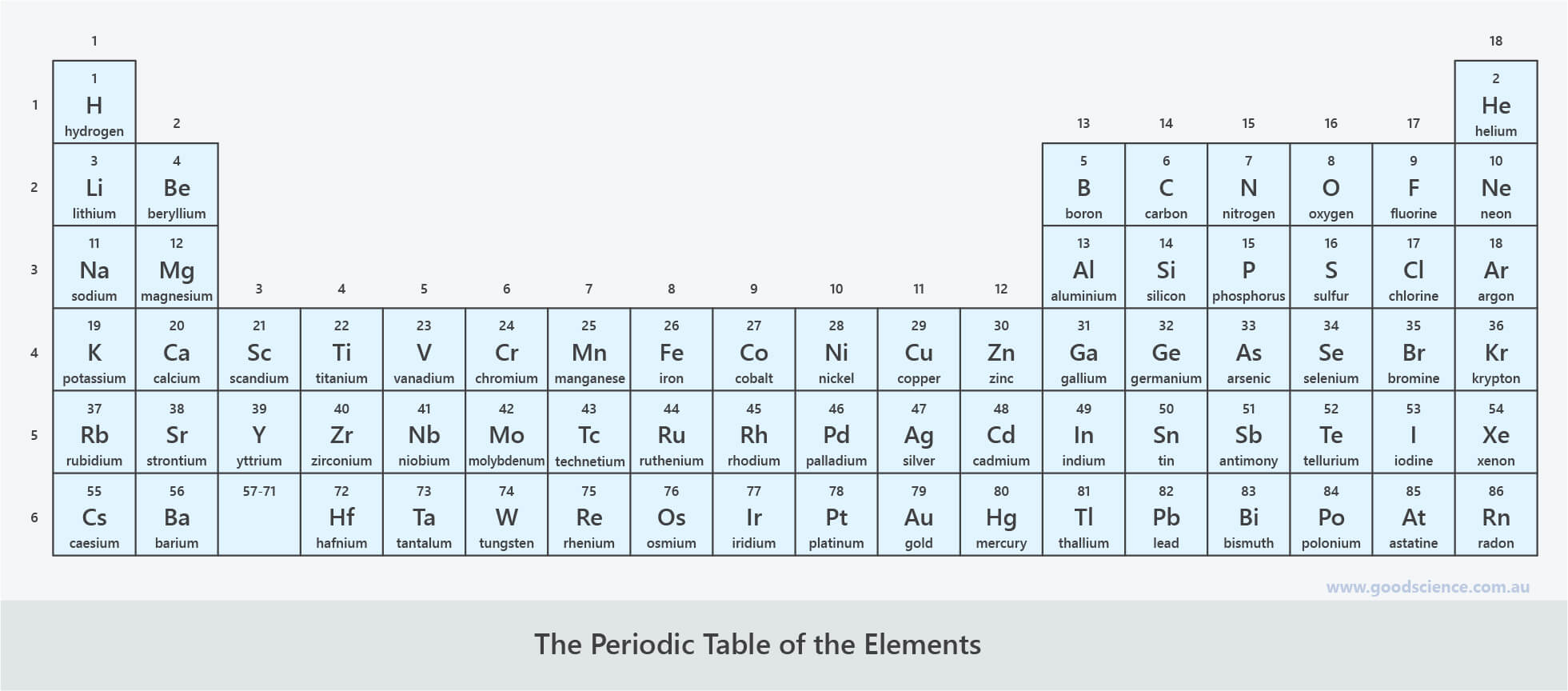
In this lesson we will learn how elements are arranged in the periodic table, according to trends in their properties.
Learning Outcomes
By the end of this lesson you will be able to:
- Describe the general layout of the periodic table.
- Identify groups and periods on the periodic table.
- Describe the distribution of solids, liquids and gases on the periodic table.
- Describe the distribution of metals, non-metals and metalloids on the periodic table.

(Image: natros, Adobe Stock)
Lesson Summary
- The periodic table is a way of organising all of the known elements.
- The elements are listed in order of increasing atomic number.
- Each cell in the periodic table includes the element’s atomic number, chemical symbol, and name.
- The layout of the periodic table reflects trends in the properties of elements.
- For example, there are general patterns in the distribution of elements based on state of matter and metallic character.
- The vertical columns of the periodic table are called groups.
- These contain elements with similar physical and chemical properties.
- The horizontal rows of the periodic table are called periods.
- These contain elements with varying physical and chemical properties.
(Header image: salita2010, Adobe Stock)
