This is a lesson summary. The full lesson can be viewed by purchasing an online course subscription.
Learning Objective
In this lesson we will learn how atoms can gain or lose electrons to form ions, and subsequently, ionic compounds.
Learning Outcomes
By the end of this lesson you will be able to:
- Describe the most stable type of electron configuration.
- Describe how metals lose electrons to form positive ions, and non-metals gain electrons to form negative ions.
- Describe how positive and negative ions combine to form ionic compounds.
- Correlate the reactivity of an element with its location on the periodic table and the type of ion it forms.
- Differentiate between monatomic and polyatomic ions.
(Image: Peter Hermes Furian, Adobe Stock)
Lesson Summary
- The most stable type of electron configuration for atoms is to have a full valence shell.
- Noble gases have full valence shells, and therefore are very unreactive.
- Most other elements are able to attain full valance shells by gaining or losing electrons.
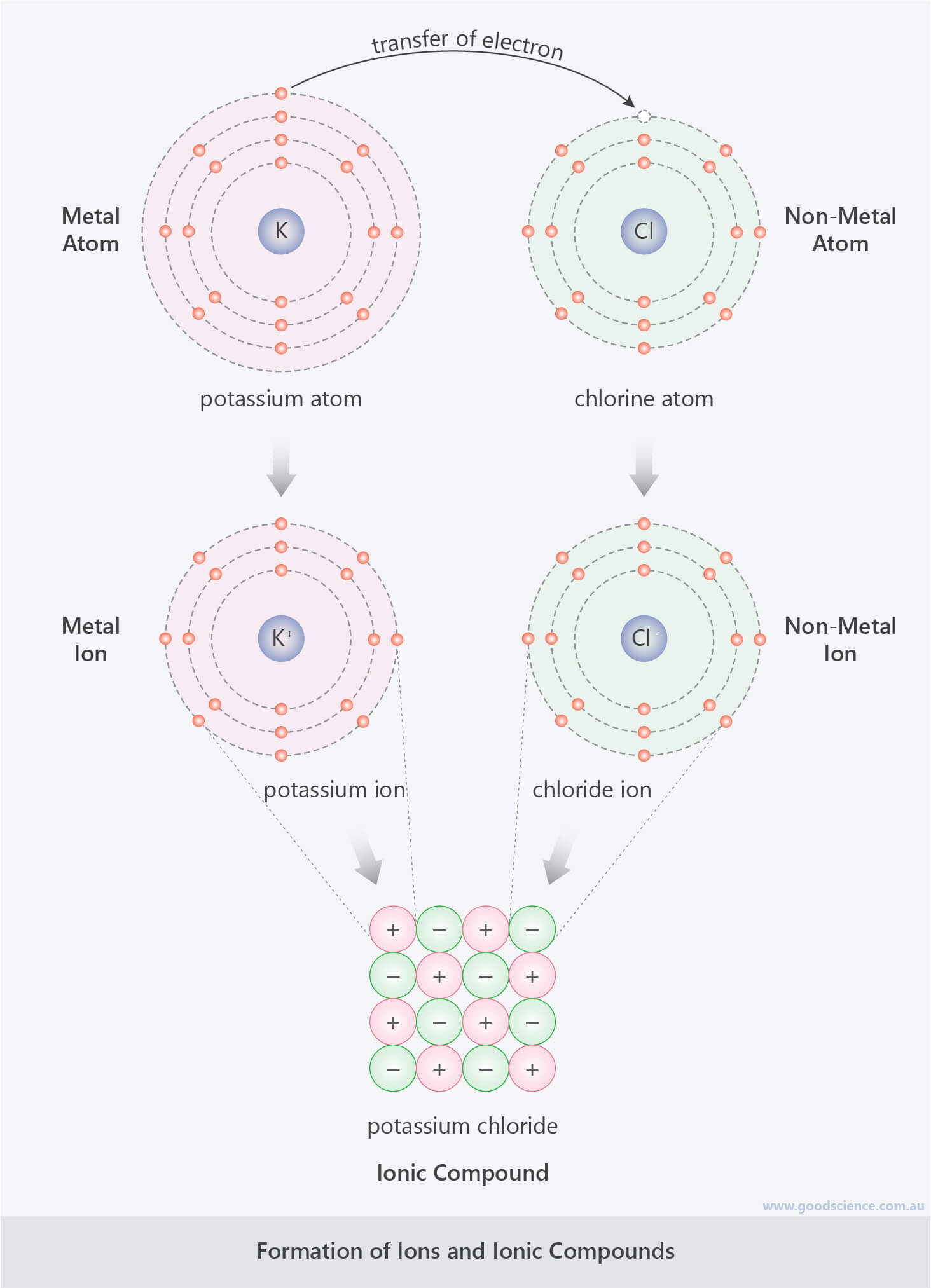
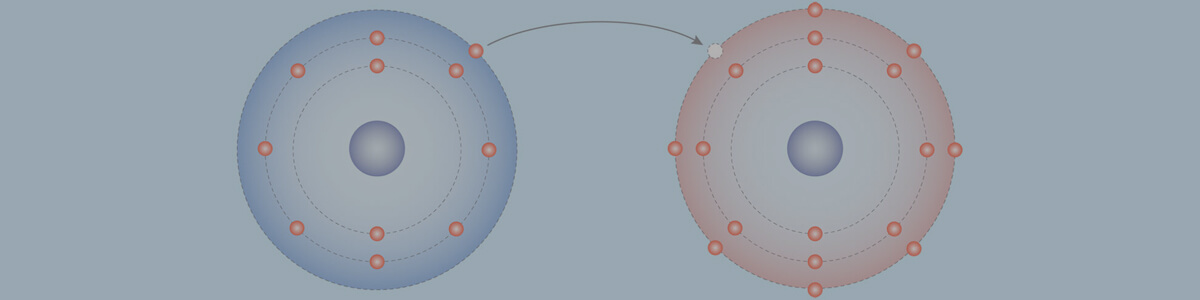
- When atoms gain or lose electrons, they form ions, which are charged particles due to the uneven numbers of protons and electrons.
- If an atom gains electrons, it forms a negative ion (anion), as there are more electrons than protons.
- If an atom loses electrons, it forms a positive ion (cation), as there are more protons than electrons.
- The size of the charge of an ion is equal to the difference in the numbers of protons and electrons.
- Atoms of group 1 elements have 1 valence electron, therefore lose 1 electron to form ions with a charge of +1.
- Atoms of group 2 elements have 2 valence electrons, therefore lose 2 electrons to form ions with a charge of +2.
- Atoms of group 13 elements have 3 valence electrons, therefore lose 3 electrons to form ions with a charge of +3.
- Atoms of group 17 elements have 7 valence electrons, therefore gain 1 electron to form ions with a charge of –1.
- Atoms of group 16 elements have 6 valence electrons, therefore gain 2 electrons to form ions with a charge of –2.
- Atoms of group 15 elements have 5 valence electrons, therefore gain 3 electrons to form ions with a charge of –3.
- For elements that form ions, the closer their atoms are to having full valence shells, the more reactive they are.
- Therefore, group 1 metals are more reactive than group 2 metals, and group 2 metals are more reactive than group 13 metals.
- Similarly, group 17 non-metals are more reactive than group 16 non-metals, and group 16 non-metals are more reactive than group 15 non-metals.
- Monatomic ions are ions derived from single atoms.
- Polyatomic ions are ions derived from multiple atoms.
- The formation of positive and negative ions occurs simultaneously.
- Metal atoms donate electrons to non-metal atoms, resulting in the formation of positive metal ions and negative non-metals ions.
- Positive and negative ions combine to form ionic compounds, which are lattice structures held together by ionic bonds between positive and negative ions.