This is a lesson summary. The full lesson can be viewed by purchasing an online course subscription.
Learning Objective
In this lesson we will learn how electrons are arranged around the nuclei of atoms.
Learning Outcomes
By the end of this lesson you will be able to:
- Describe how electrons are arranged into electron shells, and explain how these shells are filled.
- Describe how electron shells are labelled, including the valence shell.
- Draw and write the electron configuration for the first 20 elements.
- Describe the relationship between an element’s group number and the number of valence electrons in its atoms.
(Image: nikolaybarbov, Adobe Stock)
Lesson Summary
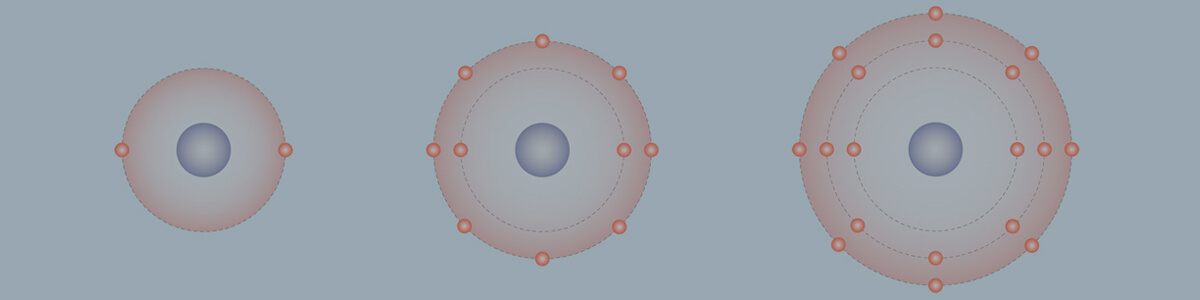
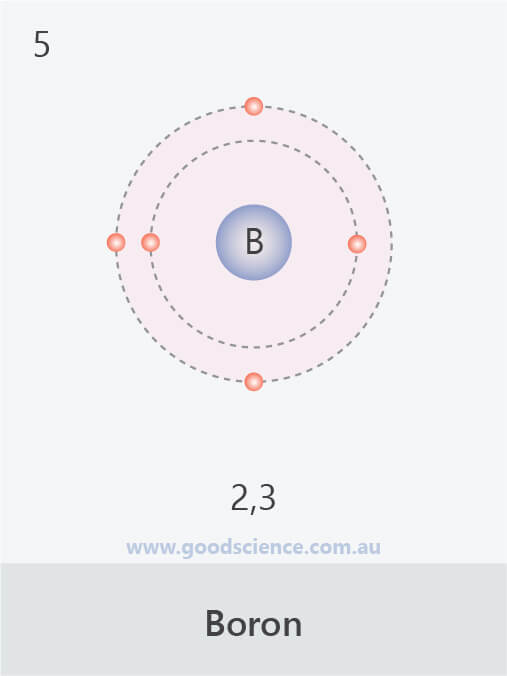
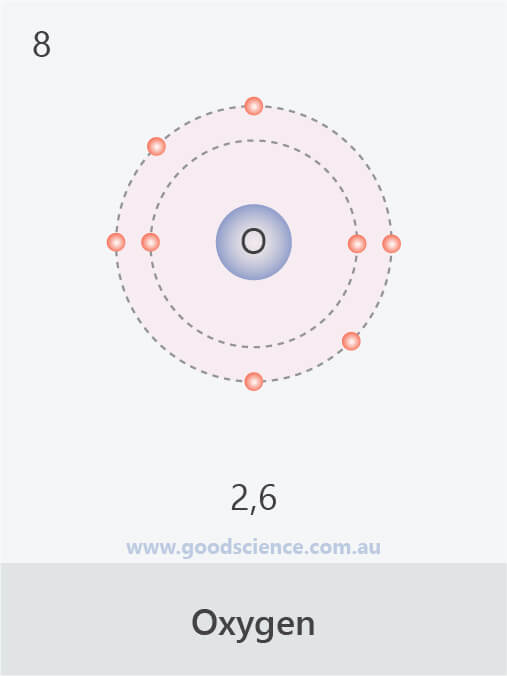
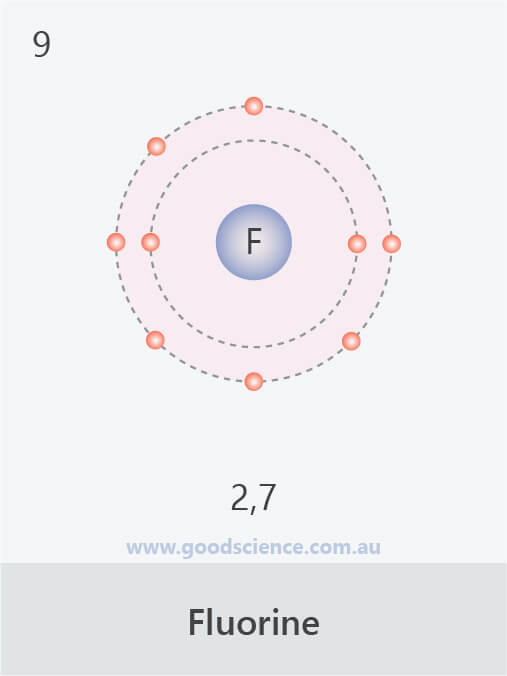
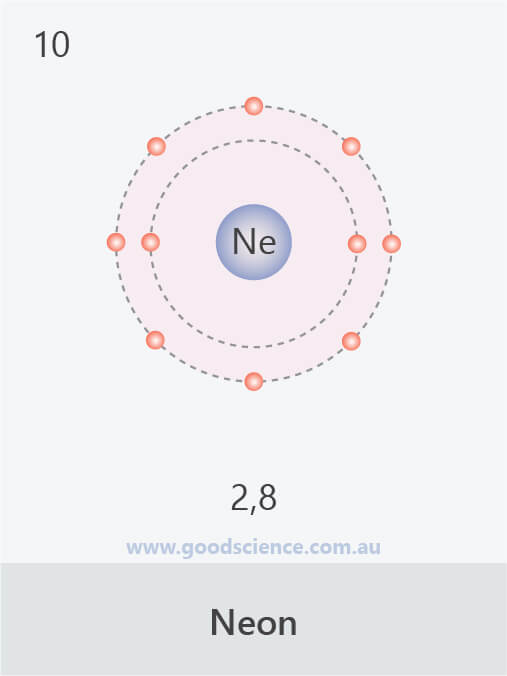
- Electrons in an atom are located in defined regions called electron shells, which surround the nucleus.
- This arrangement of electrons is referred to as the electron configuration.
- There are ‘rules’ which determine how electron shells are filled, and how many electrons they can contain:
- Inner shells begin filling first; they are smaller and can hold less electrons.
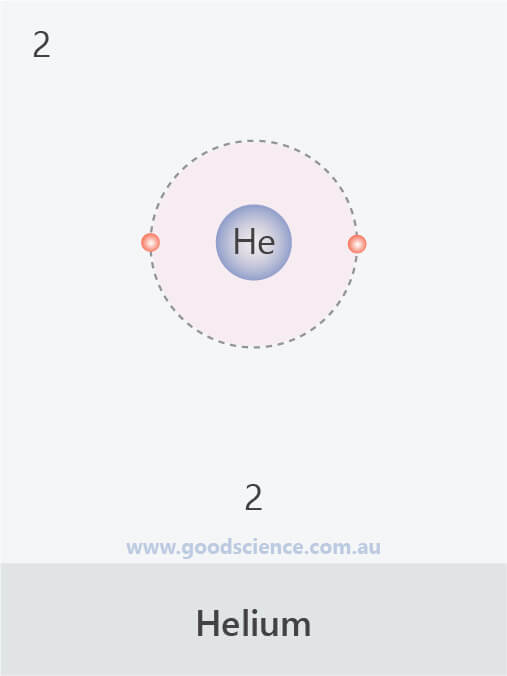
- A maximum of 2 electrons can occupy the first shell.
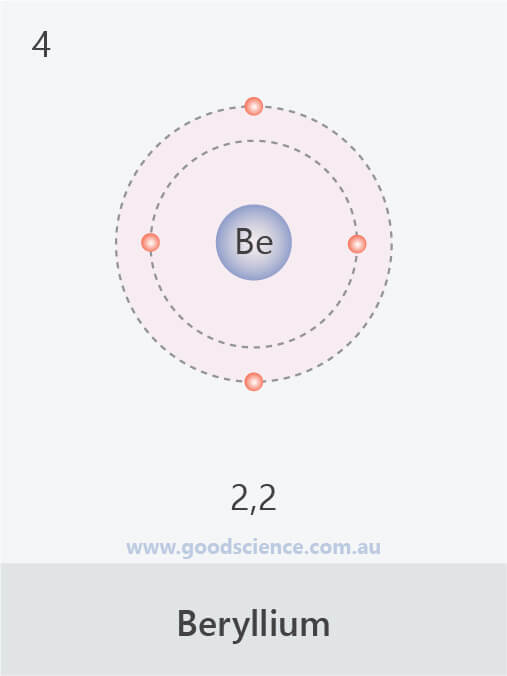
- A maximum of 8 electrons can occupy the second shell.
- A maximum of 18 electrons can occupy the third shell, but the fourth shell will begin to fill once the third shell contains 8 electrons.
- A maximum of 8 electrons can occupy the valence shell (outermost shell) of any atom, unless the valence shell is the only shell, in which case there can be a maximum of 2 electrons.
- The electron configuration of an atom can be written as the numbers of electrons in each shell, separated by a comma.




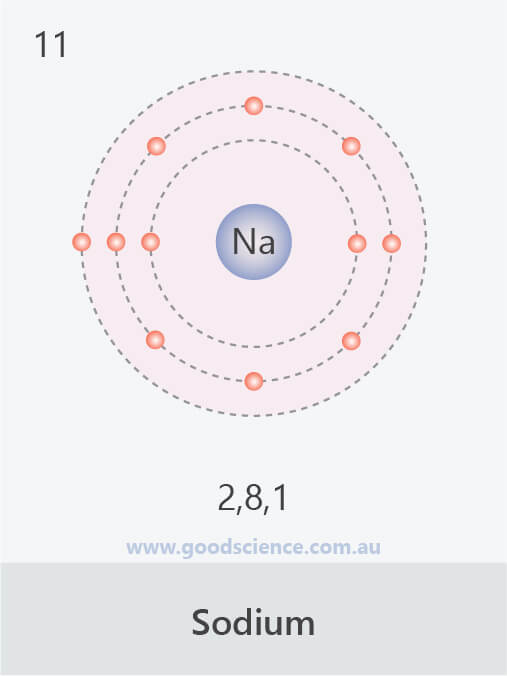
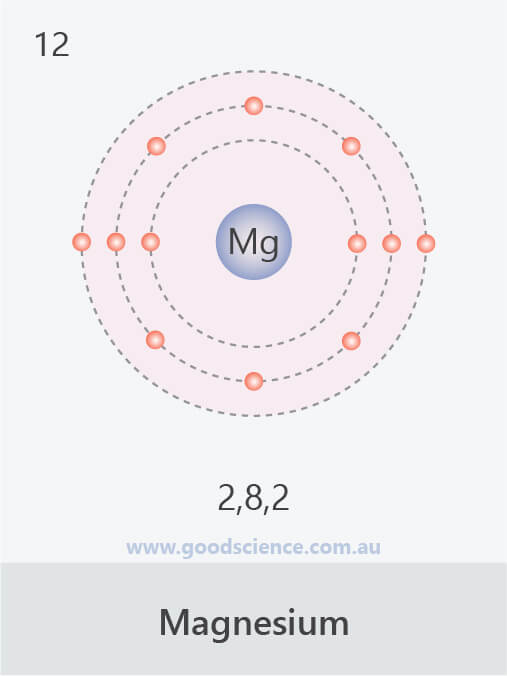
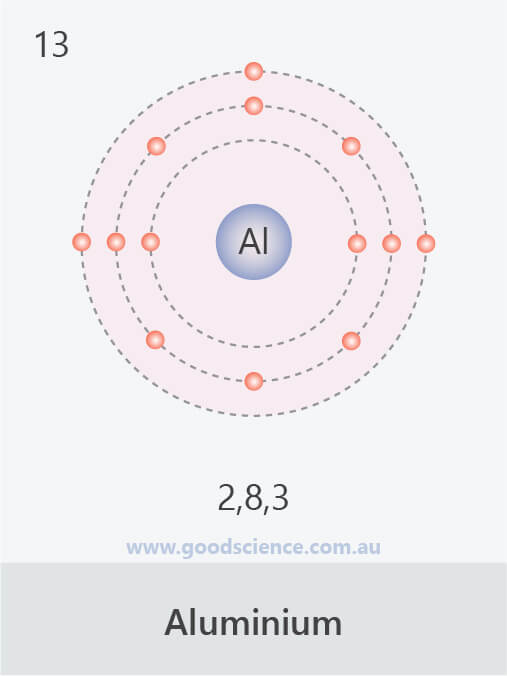
![]()


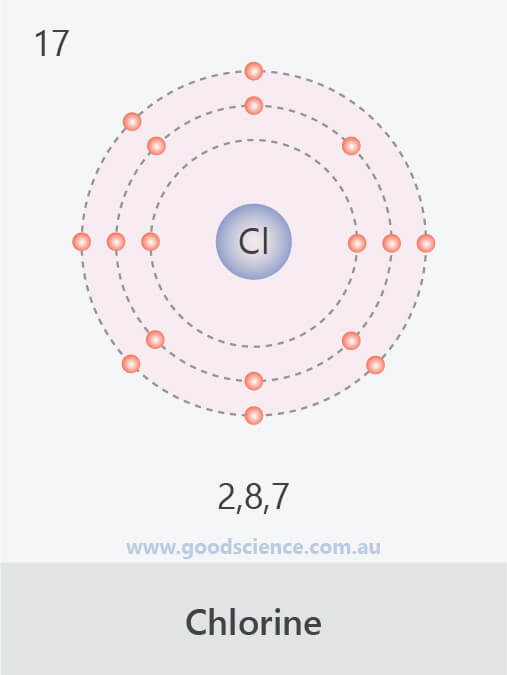
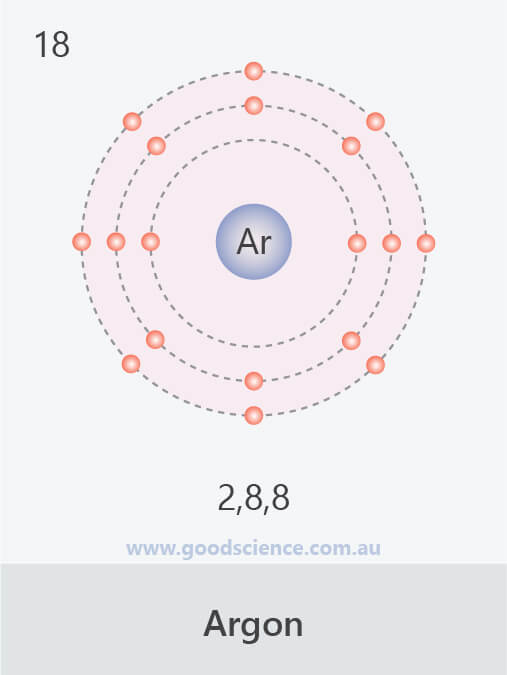

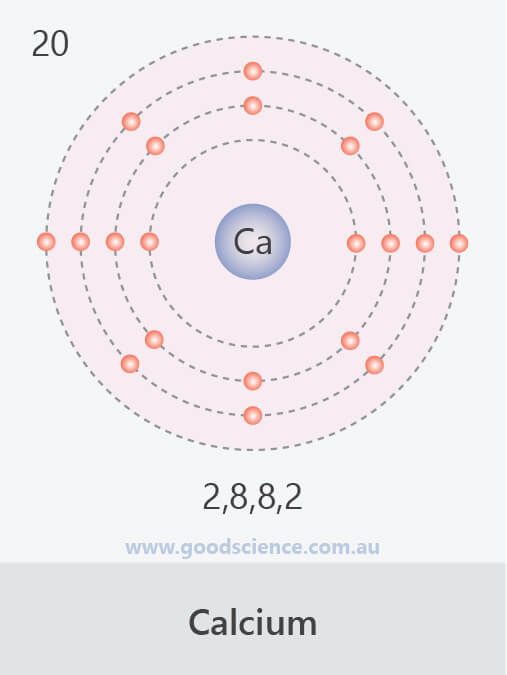
The electron configurations of the first 20 elements.