This is a lesson summary. The full lesson can be viewed by purchasing an online course subscription.
Learning Objective
In this lesson we will learn how to represent chemical reactions as word equations and formula equations.
Learning Outcomes
By the end of this lesson you will be able to:
- Identify reactants and products in chemical reactions.
- Represent chemical reactions as word equations.
- Represent chemical reactions as formula equations.
(Image: doethion, Adobe Stock)
Lesson Summary
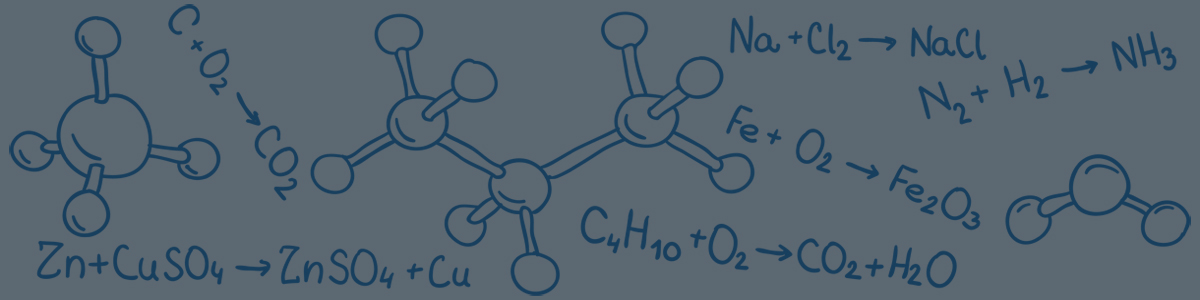
- A chemical change can also be referred to as a chemical reaction.
- A chemical reaction involves one or more substances, known as reactants, interacting to form one or more new substances, known as products.
- There are two main ways that chemical reactions can be described:
- In full sentences.
- As chemical equations.
- The general format of a chemical equation is:
- The reactants and products are separated by an arrow, which shows that a chemical change has taken place.
- There are two types of chemical equations – word equations and formula equations.
- Word equations contain the chemical names of reactants and products.
- Formula equations contain the chemical symbols of reactants and products.
(Header image: kytalpa, Adobe Stock)