This is a lesson summary. The full lesson can be viewed by purchasing an online course subscription.
Learning Objective
In this lesson we will learn about the different types of chemical bonds that can exist between atoms and the different types of chemical structures atoms can form.
Learning Outcomes
By the end of this lesson you will be able to:
- Describe metallic, ionic and covalent bonding.
- Describe properties of monatomic, metallic, ionic, covalent molecular and covalent network structures.
- Identify the type of chemical bonding in different elements and compounds.
- Explain how the type of bonding and structures an element forms is related to its location on the periodic table.

(Image: Bin im Garten, Wikimedia Commons)
Lesson Summary
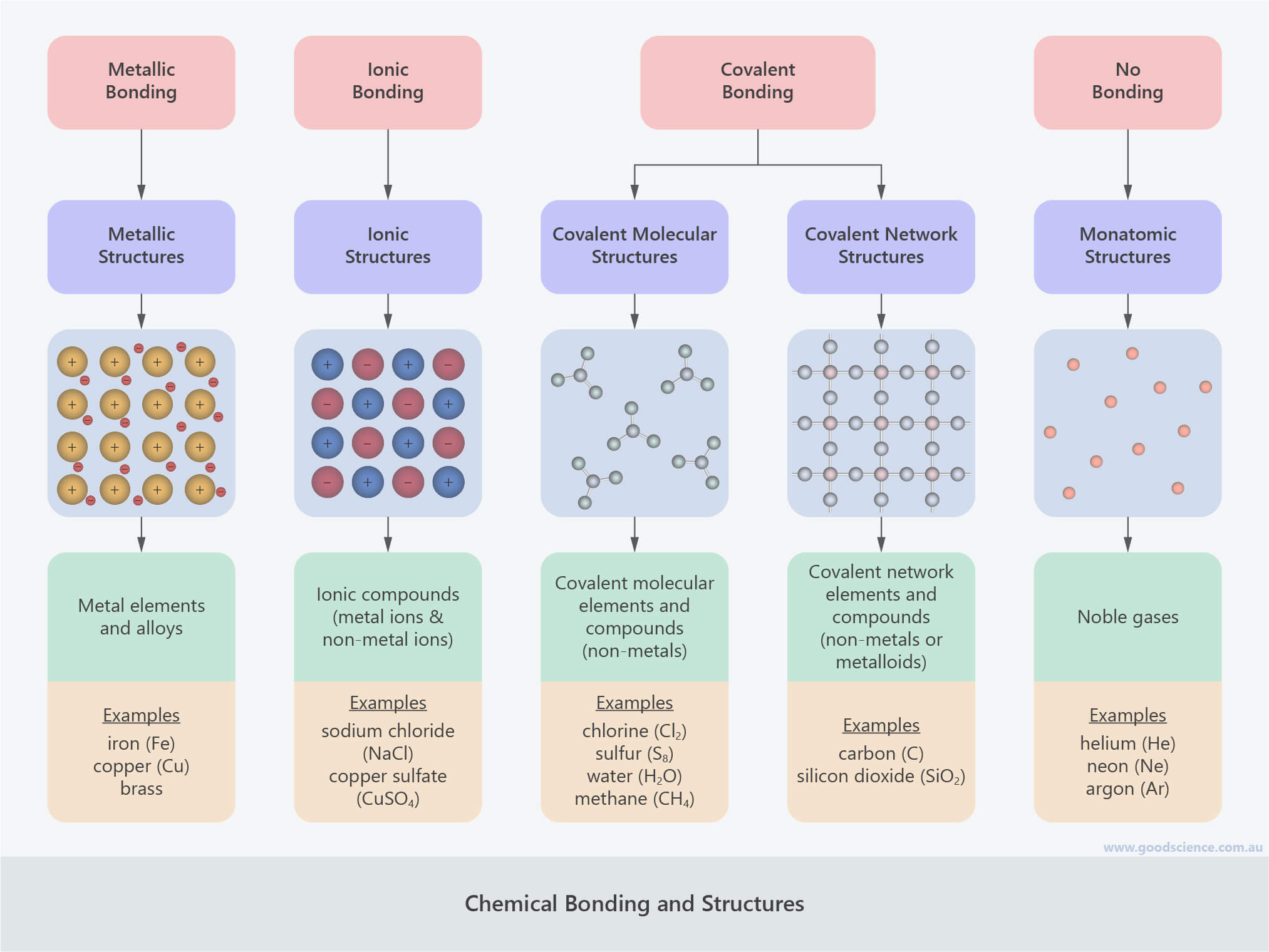
- Chemical bonds are strong forces of attraction between atoms that hold them together.
- Atoms in elements and compounds can be joined by three different types of chemical bonds:
- Metallic bonds
- Electrostatic forces of attraction between positively charged metal ions and the negatively charged electrons surrounding them.
- Ionic bonds
- Electrostatic forces of attraction between positive and negative ions.
- Covalent bonds
- Bonds involving the sharing of valence electrons between non-metal atoms.
- The different types of chemical bonds, or the lack thereof, result in five different types of chemical structures:
- Monatomic structures
- Individual atoms, with no chemical bonds between them.
- Include elements only.
- Metallic structures
- Rigid but malleable lattices of metal ions surrounded by delocalised valence electrons, connected by metallic bonds.
- Include elements and alloys.
- Ionic structures
- Hard lattices of positive and negative ions, connected by ionic bonds; composed of monatomic metal and non-metal ions, or polyatomic ions.
- Include compounds only.
- Covalent molecular structures
- Discrete molecules consisting of non-metal atoms joined by covalent bonds.
- Include elements and compounds.
- Covalent network structures
- Hard lattices of non-metal atoms connected by covalent bonds; may also involve metalloid atoms.
- Include elements and compounds.
(Header image: AlexanderAlUS, Wikimedia Commons)