This is a lesson summary. The full lesson can be viewed by purchasing an online course subscription.
Learning Objective
In this lesson we will learn how atoms are defined by the number of protons they contain, as well as the number of neutrons.
Learning Outcomes
By the end of this lesson you will be able to:
- Define atomic number.
- Define mass number.
- Compare isotopes of an element.
(Image: majcot, Adobe Stock)
Lesson Summary
- The number of protons in an atom is called the atomic number.
- Atomic Number = Number of Protons
- The number of protons plus the number of neutrons in an atom is called the mass number.
- Mass Number = Number of Protons + Number of Neutrons
- The atomic number of an element is fixed but the mass number can vary.
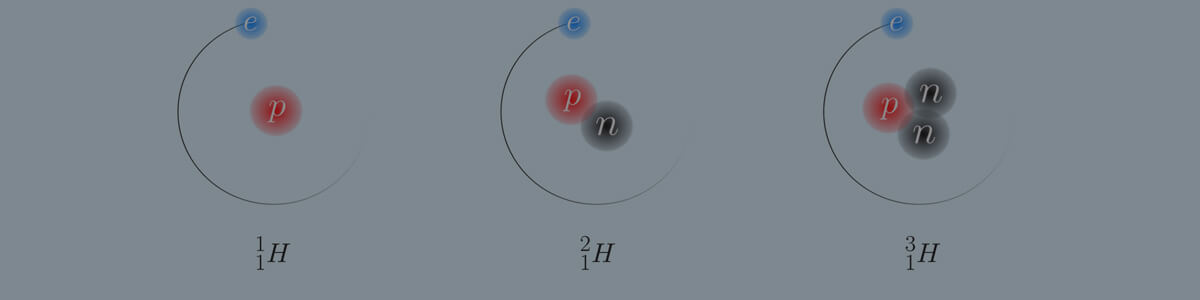
- Atoms that have the same atomic number (number of protons) but different mass numbers (number of neutrons) are called isotopes.
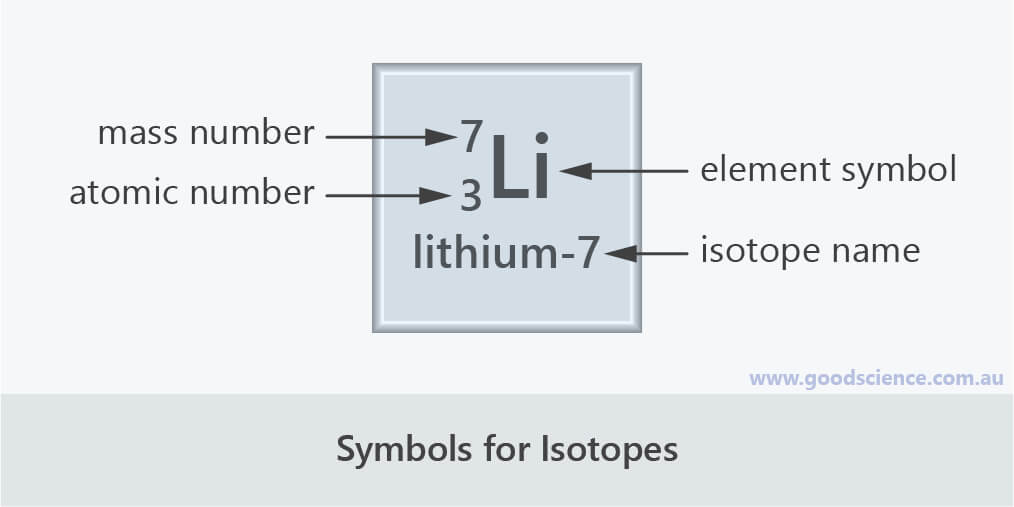
The isotope lithium-7 has the atomic number 3 and the mass number 7.
(Header image: Johannes Schneider, Wikimedia Commons)