This is a lesson summary. The full lesson can be viewed by purchasing an online course subscription.
Learning Objective
In this lesson we will compare acids, bases and neutral solutions. We will also discuss how acidity is measured and how it can be adjusted.
Learning Outcomes
By the end of this lesson you will be able to:
- Define acids, bases and neutral solutions, in terms of hydrogen ions and hydroxide ions.
- Compare the properties and uses of acids and bases.
- Describe how pH is a measure of the acidity or alkalinity of a solution, and explain how it can be adjusted.
- Describe the use of pH indicators for distinguishing between acids, bases and neutral solutions.
- Distinguish between the terms ‘strong’, ‘weak’, ‘concentrated’ and ‘dilute’, when discussing acids and bases.
(Image: robin_ph, Adobe Stock)
Lesson Summary
- Acids are substances that produce hydrogen ions (H+).
- Bases are substances that produce hydroxide ions (OH–).
- Soluble bases are called alkalis and the solutions they form are called alkaline solutions.
- Acids taste sour, while bases taste bitter.
- Common laboratory acids include hydrochloric acid (HCl), sulfuric acid (H2SO4) and nitric acid (HNO3).
- Common laboratory bases include potassium hydroxide (KOH), magnesium oxide (MgO), calcium carbonate (CaCO3) and sodium hydrogen carbonate (NaHCO3).
- A strong acid or base completely ionises in solution.
- A weak acid or bases only partially ionises in solution.
- A concentrated acid or base is a solution formed from a large number of molecules.
- A dilute acid or base is a solution formed from a small number of molecules.
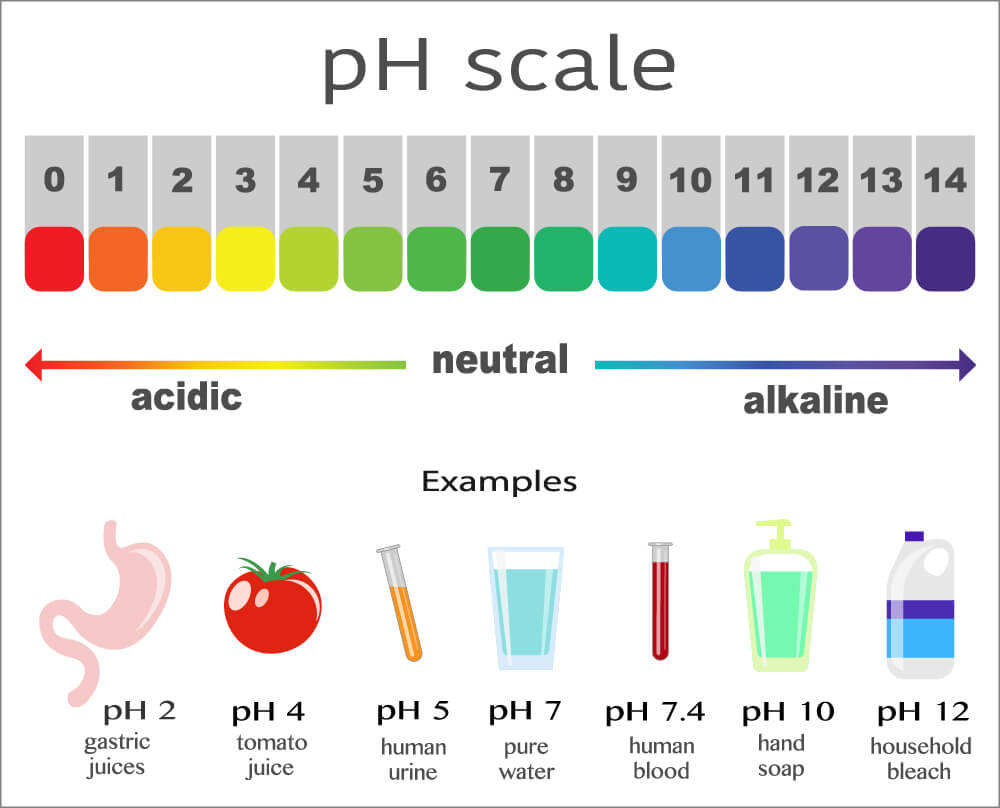
- The pH scale is a measure of how acidic or alkaline a solution is, based on the relative concentrations of hydrogen ions and hydroxide ions.
- It is a numerical scale from 0 to 14.
- Acids have a pH less than 7, and a higher proportion of hydrogen ions than hydroxide ions.
- Bases have a pH greater than 7, and a higher proportion of hydroxide ions than hydrogen ions.
- Neutral solutions have a pH equal to 7, and an equal proportion of hydrogen ions and hydroxide ions.
- The further away from 7 the pH of a solution is, the greater the difference between hydrogen ion and hydroxide ion concentration, and the more acidic or alkaline the solution.
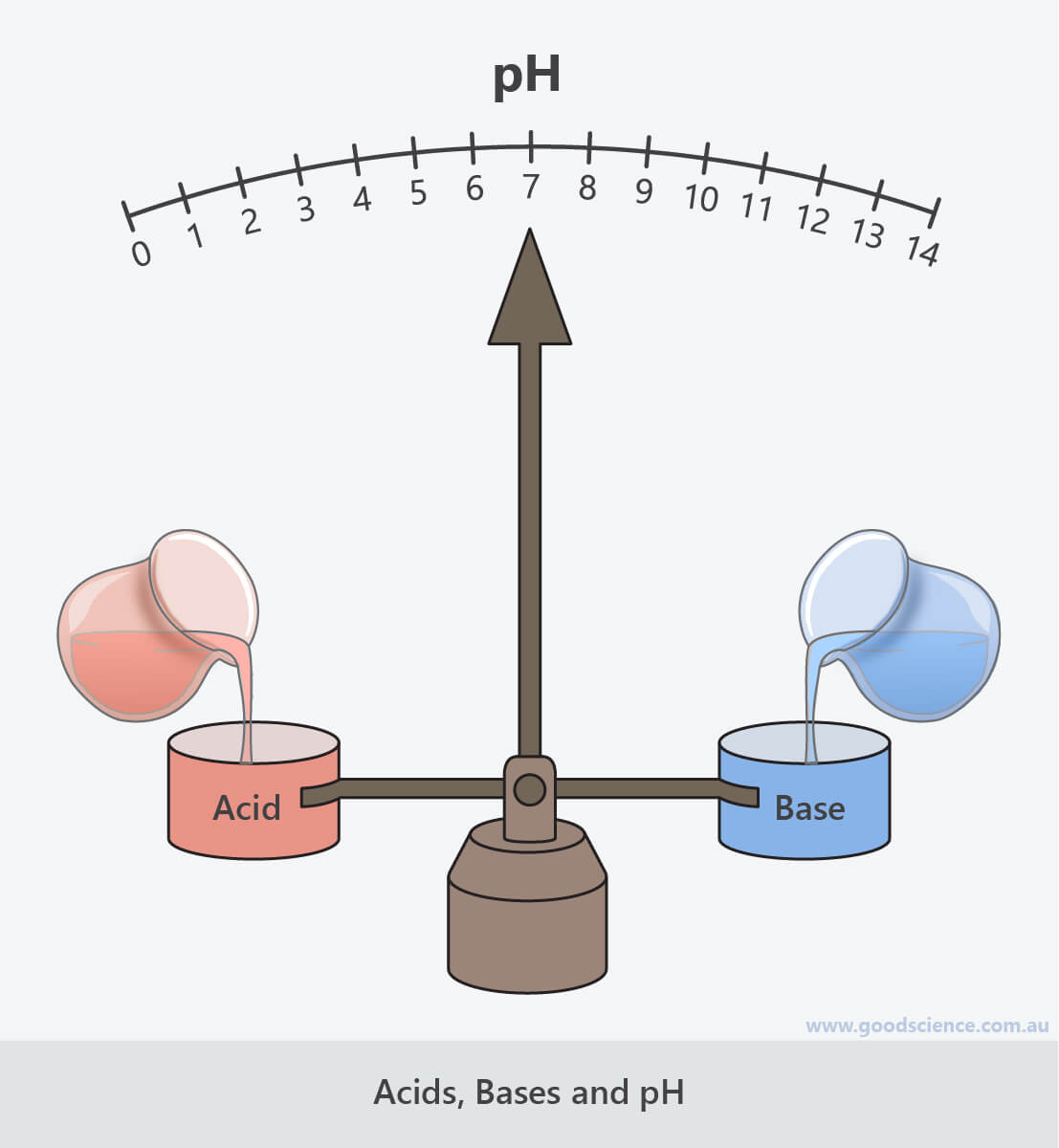
- The pH of a solution can be increased by adding base.
- The pH of a solution can be decreased by adding acid.
- Equal amounts of hydrogen ions and hydroxide ions in a solution results in neutralisation.
- pH indicators are substances that change colour depending on the pH of a solution.
- Red litmus paper turns blue if placed in an alkaline solution, but remains red if placed in an acidic or neutral solution.
- Blue litmus paper turns red if placed in an acidic solution, but remains blue if placed in an alkaline or neutral solution.
- Universal indicator produces a range of colours, depending on the pH of a solution.
- It can distinguish between strong and weak acids and bases, and be used to determine the approximate pH value of a solution.
- pH meters can electronically measure the pH value of a solution.
(Header image: Kuebi, Wikimedia Commons)
