This is a lesson summary. The full lesson can be viewed by purchasing an online course subscription.
Learning Objective
In this lesson we will learn about the layout of the periodic table and how it relates to the electron configuration of elements.
Learning Outcomes
By the end of this lesson you will be able to:
- Describe the general layout of the periodic table.
- Identify different groups and periods on the periodic table.
- Describe some common properties of elements in particular groups, including alkali metals, alkaline earth metals, halogens and noble gases.
- Describe the relationship between the electron configuration of an element and its location on the periodic table.
- Describe periodic trends in atom size and chemical reactivity.
(Image: concept w, Adobe Stock)
Lesson Summary
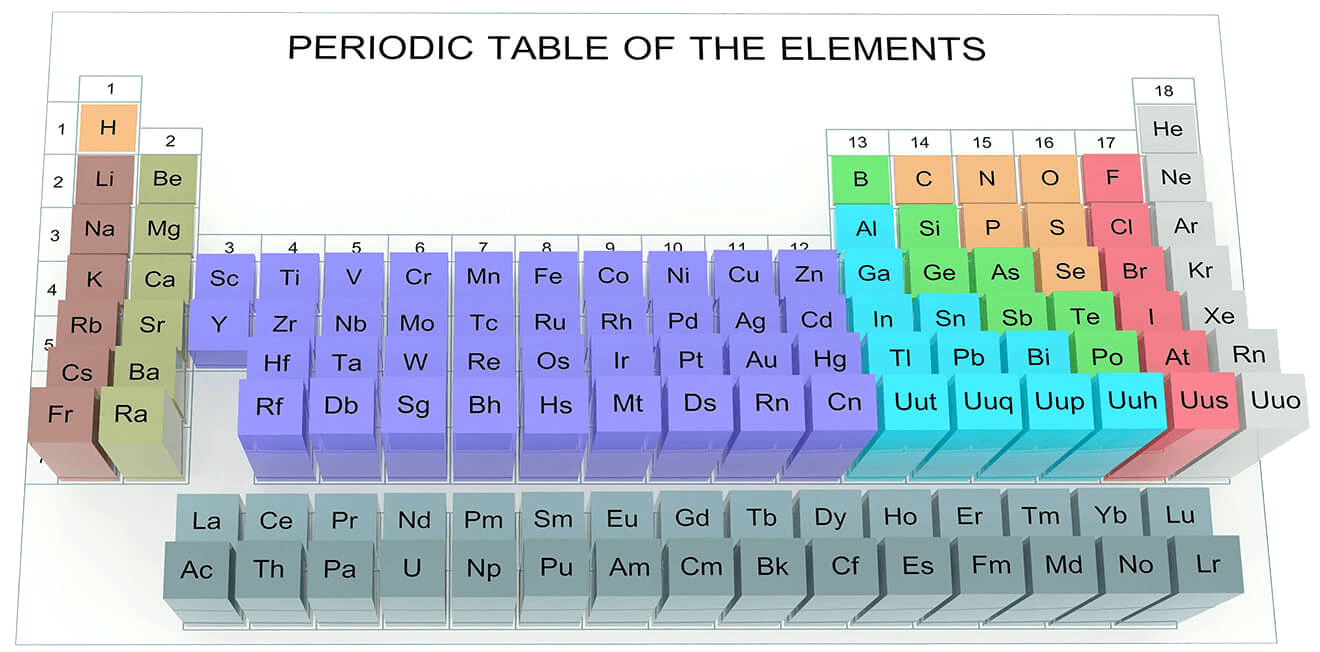
- The periodic table of the elements lists all the known elements, in a way that reflects periodic trends in their physical and chemical properties.
- Elements are listed in order of increasing atomic number (proton number) and arranged into groups (columns) and periods (rows).
- The layout of the periodic table arranges groups so that they contain elements with similar physical and chemical properties.
- These include alkali metals, alkaline earth metals, halogens and noble gases.
- The layout also reflects patterns in the electron configuration of elements.
- Main groups contain elements with the same number of valence electrons, therefore, they form ions with the same charge.
- Periods contain elements with the same number of electron shells.
- Atom size increases down groups and decreases across periods.
- For metals, reactivity increases down groups and decreases towards the centre of the periodic table (excluding transition metals).
- For non-metals, reactivity decreases down groups and decreases towards the centre of the periodic table (excluding noble gases).
(Image: Jynto, Wikimedia Commons)
(Header image: Tomasz Zajda, Adobe Stock)
